Proton Pump Inhibitors: How to Deprescribe These Nutrient Robbers
Many medications can cause nutritional deficiencies, and proton pump inhibitors (PPIs) are no different.
First is magnesium deficiency. Several reports have linked long-term PPI use with an increased risk of hypomagnesemia, especially over a year.1 Results of studies suggest that PPIs inhibit active transport of magnesium in the intestine.1 Severe hypomagnesemia can potentially lead to serious adverse effects including arrhythmias, hypocalcemia, hypokalemia, hypoparathyroidism, muscle spasms, and seizures. Suggested supplementation of magnesium is 250 to 400 mg/day.2
Second is vitamin B12 rated as a moderate depletion. Monitoring of vitamin B12 is recommended, and some people may need a supplement.1Unless PPI use is prolonged (2 years or more) or dietary vitamin intake is low, clinically significant vitamin B12deficiency is unlikely. Furthermore, vitamin B12 deficiency is more likely in individuals taking high doses of PPIs. The good news is that vitamin B12deficiency is expected to diminish after patients discontinue PPI therapy.1
Because gastric acid is needed to release vitamin B12 from protein for absorption, PPIs can reduce the absorption of protein-bound (dietary) vitamin B12.1 Suggested supplementation of vitamin B12 is 25 to 400 μg/day.2
In addition, the Natural Medicines Comprehensive Database rates dibencozide as a moderate depletion.1
For details on other depletions induced by PPIs and H2blockers, see the Table.1
Growing Concern
It is common for PPIs to be continued for prolonged periods and, in some cases, indefinitely. But long-term use of PPIs can lead to adverse effects, drug interactions, misuse or overuse, and prescribing cascades.3
In addition to nutrient depletion, PPIs have been linked to an increased risk of Clostridium difficile and pneumonia infections, kidney damage, and osteoporotic fractures.4
Deprescribing
There are several methods for deprescribing PPIs, including stopping (abrupt discontinuation or tapering), stepping down (ceasing taking medication, followed by H2blocker therapy), and reducing (intermittent PPI use, on-demand PPI use, or lowering the dose).5 Regardless of the approach, the overall goal of deprescribing is to avoid adverse effects, improve or maintain quality of life, and reduce inappropriate medicine use.3,5
Although there have been small studies that have demonstrated successful deprescribing methods, there had been no guidelines to describe the benefits and drawbacks of deprescribing until now.3
Evidence-based guidelines to help clinicians deprescribe PPIs were published in the Canadian Family Physicianjournal in 2017.5 These guidelines used a systematic review of deprescribing trials and examined reviews regarding the harm associated with the continued use of PPIs. The development of these guidelines involved a team comprising a family physician, a gastroenterologist, 3 pharmacists, and 5 nonvoting members.5
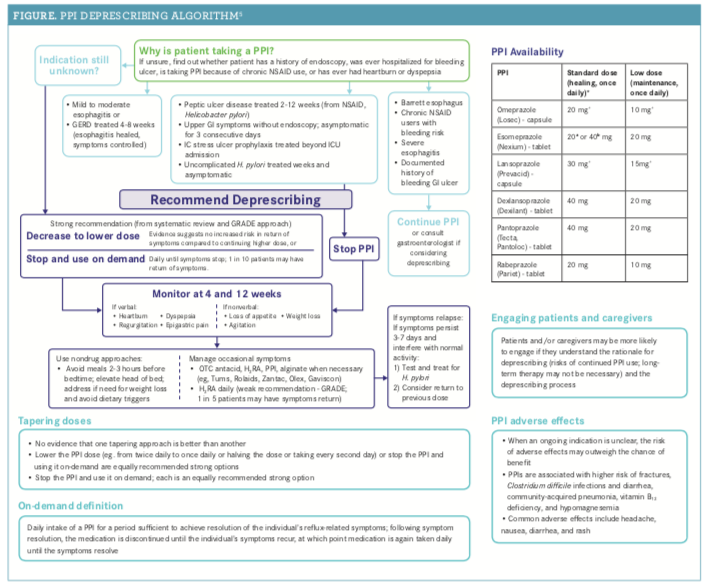
The guidelines are designed to help clinicians make decisions about how and when to deprescribe PPIs. They are meant to be used in conjunction with treatment guidelines for gastroesophageal reflux disease (GERD) and peptic ulcer disease, and they take into account a patient’s personal situation and are not meant to dictate decision making, the authors noted.5For the PPI deprescribing algorithm, see the Figure.5
On-demand PPI use is defined as taking a PPI for a period that is sufficient to achieve symptom resolution. Upon symptom resolution, the PPI is to be discontinued until the symptoms recur, in which case, the PPI will again be taken daily until symptoms resolve.5 These recommendations are not for individuals with Barrett esophagus, a history of bleeding GI ulcers, or severe esophagitis (grade C or D, as defined by the Los Angeles Classification for the endoscopic assessment of reflux esophagitis), the authors noted.5
In addition, there is a recently published Cochrane systematic review that may be of value when considering deprescribing PPIs.3
The authors noted that there are several situations in which continued use of PPIs may be needed and listed those in the algorithm. Furthermore, they recommended seeking advice from a gastroenterologist to assess continuing risk factors for GI disease.5
Many of the deprescribing trials included follow-up post deprescribing. Because of this, the investigators recommended establishing a post-deprescribing plan that includes a follow-up after 4 weeks to assess symptom control and after 12 weeks to assess symptoms, the frequency of on-demand use, and other factors, as well as to reassess the need to go back on continuous treatment.5
Anyssa Garza, PharmD, BCMAS, received her doctor of pharmacy degree from The University of Texas at Austin. She is the vice president of Content and Patient Education Programs at Digital Pharmacist and an adjunct assistant professor at The University of Texas at Austin College of Pharmacy.
References
1. Nutrient depletion. Natural Medicines Comprehensive Database website. naturaldatabase.com. Accessed June 12, 2018.
2. Common drug classes, drug-nutrient depletions, & drug-nutrient interactions. Nature Made website. naturemade.com/~/media/Images/NatureMade/PDF/Health%20Care%20Professionals/HCP%20Updates%20042315/Common%20Drug%20Classes%20and%20Nutrient%20Interactions%20Chart%20FNL.ashx. Published 2015. Accessed May 6, 2018.
3. Boghossian TA, Rashid FJ, Thompson W, et al. Deprescribing versus continuation of chronic proton pump inhibitor use in adults. Cochrane Database Syst Rev.2017;3:CD011969. doi: 10.1002/14651858.CD011969.pub2.
4. Yu LY, Sun LN, Zhang XH, et al. A review of the novel application and potential adverse effects of proton pump inhibitors. Adv Ther.2017;34(5):1070-1086. doi: 10.1007/s12325-017-0532-9.
5. Farrell B, Pottie K, Thompson W, et al. Deprescribing proton pump inhibitors: evidence-based clinical practice guideline. Can Fam Physician.2017;63(5):354-364.
First is magnesium deficiency. Several reports have linked long-term PPI use with an increased risk of hypomagnesemia, especially over a year.1 Results of studies suggest that PPIs inhibit active transport of magnesium in the intestine.1 Severe hypomagnesemia can potentially lead to serious adverse effects including arrhythmias, hypocalcemia, hypokalemia, hypoparathyroidism, muscle spasms, and seizures. Suggested supplementation of magnesium is 250 to 400 mg/day.2
Second is vitamin B12 rated as a moderate depletion. Monitoring of vitamin B12 is recommended, and some people may need a supplement.1Unless PPI use is prolonged (2 years or more) or dietary vitamin intake is low, clinically significant vitamin B12deficiency is unlikely. Furthermore, vitamin B12 deficiency is more likely in individuals taking high doses of PPIs. The good news is that vitamin B12deficiency is expected to diminish after patients discontinue PPI therapy.1
Because gastric acid is needed to release vitamin B12 from protein for absorption, PPIs can reduce the absorption of protein-bound (dietary) vitamin B12.1 Suggested supplementation of vitamin B12 is 25 to 400 μg/day.2
In addition, the Natural Medicines Comprehensive Database rates dibencozide as a moderate depletion.1
For details on other depletions induced by PPIs and H2blockers, see the Table.1
Growing Concern
It is common for PPIs to be continued for prolonged periods and, in some cases, indefinitely. But long-term use of PPIs can lead to adverse effects, drug interactions, misuse or overuse, and prescribing cascades.3
In addition to nutrient depletion, PPIs have been linked to an increased risk of Clostridium difficile and pneumonia infections, kidney damage, and osteoporotic fractures.4
Deprescribing
There are several methods for deprescribing PPIs, including stopping (abrupt discontinuation or tapering), stepping down (ceasing taking medication, followed by H2blocker therapy), and reducing (intermittent PPI use, on-demand PPI use, or lowering the dose).5 Regardless of the approach, the overall goal of deprescribing is to avoid adverse effects, improve or maintain quality of life, and reduce inappropriate medicine use.3,5
Although there have been small studies that have demonstrated successful deprescribing methods, there had been no guidelines to describe the benefits and drawbacks of deprescribing until now.3
Evidence-based guidelines to help clinicians deprescribe PPIs were published in the Canadian Family Physicianjournal in 2017.5 These guidelines used a systematic review of deprescribing trials and examined reviews regarding the harm associated with the continued use of PPIs. The development of these guidelines involved a team comprising a family physician, a gastroenterologist, 3 pharmacists, and 5 nonvoting members.5
The guidelines are designed to help clinicians make decisions about how and when to deprescribe PPIs. They are meant to be used in conjunction with treatment guidelines for gastroesophageal reflux disease (GERD) and peptic ulcer disease, and they take into account a patient’s personal situation and are not meant to dictate decision making, the authors noted.5For the PPI deprescribing algorithm, see the Figure.5
On-demand PPI use is defined as taking a PPI for a period that is sufficient to achieve symptom resolution. Upon symptom resolution, the PPI is to be discontinued until the symptoms recur, in which case, the PPI will again be taken daily until symptoms resolve.5 These recommendations are not for individuals with Barrett esophagus, a history of bleeding GI ulcers, or severe esophagitis (grade C or D, as defined by the Los Angeles Classification for the endoscopic assessment of reflux esophagitis), the authors noted.5
In addition, there is a recently published Cochrane systematic review that may be of value when considering deprescribing PPIs.3
The authors noted that there are several situations in which continued use of PPIs may be needed and listed those in the algorithm. Furthermore, they recommended seeking advice from a gastroenterologist to assess continuing risk factors for GI disease.5
Many of the deprescribing trials included follow-up post deprescribing. Because of this, the investigators recommended establishing a post-deprescribing plan that includes a follow-up after 4 weeks to assess symptom control and after 12 weeks to assess symptoms, the frequency of on-demand use, and other factors, as well as to reassess the need to go back on continuous treatment.5
Anyssa Garza, PharmD, BCMAS, received her doctor of pharmacy degree from The University of Texas at Austin. She is the vice president of Content and Patient Education Programs at Digital Pharmacist and an adjunct assistant professor at The University of Texas at Austin College of Pharmacy.
References
1. Nutrient depletion. Natural Medicines Comprehensive Database website. naturaldatabase.com. Accessed June 12, 2018.
2. Common drug classes, drug-nutrient depletions, & drug-nutrient interactions. Nature Made website. naturemade.com/~/media/Images/NatureMade/PDF/Health%20Care%20Professionals/HCP%20Updates%20042315/Common%20Drug%20Classes%20and%20Nutrient%20Interactions%20Chart%20FNL.ashx. Published 2015. Accessed May 6, 2018.
3. Boghossian TA, Rashid FJ, Thompson W, et al. Deprescribing versus continuation of chronic proton pump inhibitor use in adults. Cochrane Database Syst Rev.2017;3:CD011969. doi: 10.1002/14651858.CD011969.pub2.
4. Yu LY, Sun LN, Zhang XH, et al. A review of the novel application and potential adverse effects of proton pump inhibitors. Adv Ther.2017;34(5):1070-1086. doi: 10.1007/s12325-017-0532-9.
5. Farrell B, Pottie K, Thompson W, et al. Deprescribing proton pump inhibitors: evidence-based clinical practice guideline. Can Fam Physician.2017;63(5):354-364.
https://www.pharmacytimes.com/publications/issue/2018/july2018/proton-pump-inhibitors-how-to-deprescribe-these-nutrient-robbers-